Acidic solutions have H3O and basic solutions have OH-. PH -log 10 a H Where a is the activity.
Therefore if we move from 7 down the pH scale toward 0 the concentration of.

. Last Updated on Fri 07 Jan 2022 Medical Physiology. A solution of hydrochloric acid with a concentration of 2 gdm 3 has a pH of 13. The pH of solution is defined as - logH.
If the pH of a solution increases from pH 50 to pH 80 what happens to the concentration of hydrogen ions in the solution. At the neutral point of water both the Hydrogen ion and. Hydrogen ion concentration increases 1000 times.
A decrease in pH means more hydrogen ion and an increase of pH means less hydrogen ions. Then the pH is the logarithm of the inverse of the hydrogen ion concentration. PH is determined as the -log H so pH is negative logarithm of base 10 of hydrogen ions.
As the concentration of H increases. Thus Figure 30-3 shows that the alveolar ventilation rate. The hydrogen ion concentration decreases by a factor of 10 so the pH increases by 1 from 16 to 26.
If the hyrdoxide ion concentartion increases what happens to the hydrogen ion concentration. Apr 17 2017 It will decrease by 2. What will happen to the pH of a substance if the concentration of hydrogen H ions increases from 10 108 to 10 106.
Having more H3O- in a solution makes a solution more acidic. Where H IS CHCI d What happens to pH as the hydrogen ion concentration decreases. This means if there is a change in pH in one unit the pH concentration will change by 10 fold.
For H 108 pH 8 For H 106 pH 6 Answer link. Not only does the alveolar ventilation rate influence H concentration by changing the Pco2 of the body fluids but the H concentration affects the rate of alveolar ventilation. The pH of a solution is 40.
Chemistry Acids and Bases pH 1 Answer Monzur R. As the H ion decreases the OH- ion increase. The hydrogen ions there are.
Rosariomividaa3 and 18 more users found this answer helpful. What happens to blood pH as the concentration of lactic acid increases. As the hydronium ion concentration increases what about the pH.
The Keq for water is 10-14 This means that the Hydrogen ion concentration times the Hydroxide concentration must always be the same 10-14 H xx OH- 10-14 This means that the two ions are inversely proportional. Since the concentration of the hydrogen ions is often very low ion activity is considered as equal to the concentration of hydrogen ions. If one goes up the other goes down.
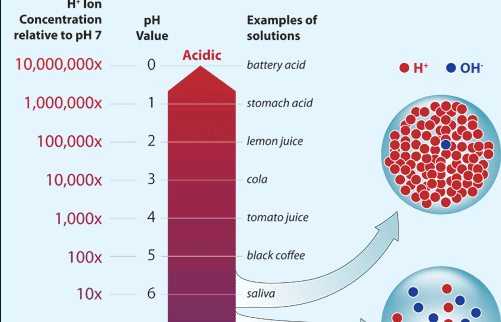
The concentration of hydrogen ions can vary across many orders of magnitudefrom 1 to 000000000000001 moles per literand we express acidity on a logarithmic scale called the pH scale. This happens when an acid is introduced. What is commonly referred to as acidity is the concentration of hydrogen ions H in an aqueous solutionSome common examples are shown in the figure at left.
As the pH increases the H ion concentration decreases and OH- ion concentration increases. O pH decreases pH increases O e Determine the hydrogen ion concentration of a solution with pH-32. See the answer See the answer done loading.
How Is The Ph Of A Solution Related To The H3oThe pH of a solution is related to H3O because that is what makes a solution acidic. The hydrogen ion concentration in a solution with a pH of 3 is two times greater than the hydrogen ion concentration in a solution with a pH of 5. If the hydrogen concentration increases the pH decreases causing the solution to become more acidic.
H 610 M Round to six decimal places as needed Determine the hydrogen ion concentration of a solution with pH 74. PH scale based on the Hydrogen ion concentration. The blood pH decreases.
The concentration of lactic acid increases. It is an inverse relationship. Why do you think the blood pH stayed neutral while the same amount of lactic acid in water was very.
PH is a measure of hydrogen ion concentration H which actually exist as H3O Hydronium. Hydrogen ion concentration increases 3 times. Hydrogen ion concentration decreases 3 times.
Increased Hydrogen Ion Concentration Stimulates Alveolar Ventilation. As the Ph increases the hydrogen ion concentration decreases. If the hydrogen concentration decreases the pH increases resulting in a solution that is less acidic and more basic.
What should be the change in the hydrogen ion concentration of the solution if its pH is to be increased to 50. -you divide 10 10-14 the H3O amount.

Lesson Explainer The Ph Scale Nagwa

Acids And Bases Acids And Bases Dissociation Of Water Into Hydrogen And Hydroxide Ions Ph Is Related To The Concentration Of Hydrogen And Hydroxide Ppt Download
Small Drop In Ph Means Big Change In Acidity Woods Hole Oceanographic Institution

0 Comments